If your immune system doesn’t work at full strength, you’re at higher risk for getting an infection or disease like Covid-19. It may not be your first thought to join a clinical trial that is studying new medicines or vaccines, but this type of research is key to protecting people who are immunocompromised.
What does “immunocompromised” mean?
Your immune system is designed to fight off infections and diseases. People who are “immunocompromised” have weakened immune systems. They may have a harder time fighting off viruses, bacteria and fungi that cause health problems.
What causes weakened immunity?
There are many reasons why your immune system might be weak, including:
- Health conditions, such as
- HIV/AIDS
- Lupus
- Rheumatoid arthritis
- Type 1 diabetes
- Primary immune deficiency
- Blood cancers, such as leukemia and lymphoma
- Certain medicines or treatments, including chemotherapy
- Organ or stem cell transplants
- Age
- Smoking
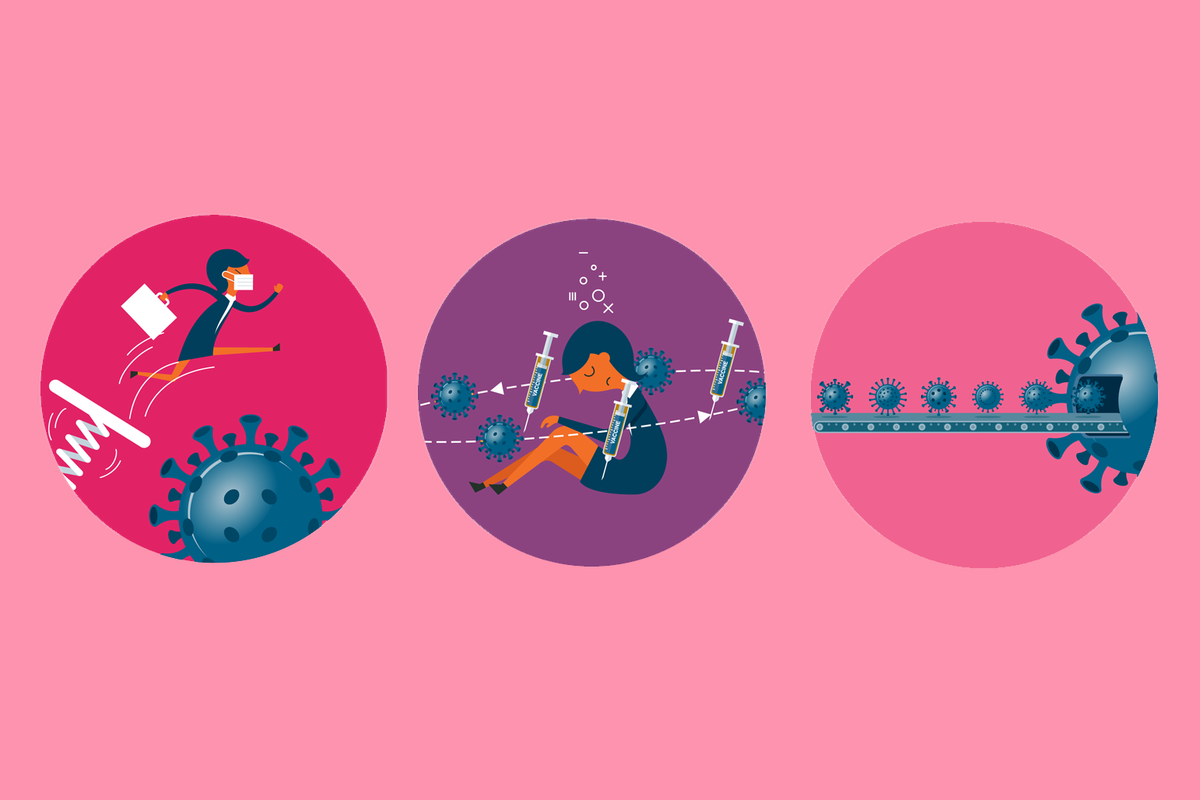
Covid-19 threats to people with weakened immune systems
- People with weak immune systems are at greater risk of getting Covid-19. They’re also more likely to get sicker if they get infected.
- Vaccines may not work as well in people whose immune systems are weak.
- Viruses evolve. New strains, called variants, of Covid-19 keep emerging. Existing medicines and vaccines may not work as well on these new variants. So, people who are immunocompromised will be at even higher risk.
How to protect immunocompromised people from Covid-19
- Healthy Habits – Improve air filtration and ventilation, spend time outdoors, increase space and distance from others when you can, wear masks and maintain hygiene practices.
- Personal health – Be on the lookout for symptoms if you've been exposed and contact your healthcare provider (HCP) quickly if you test positive.
- Research – To find new and innovative ways to protect people from Covid-19, researchers are studying new medicines and vaccines that may help immunocompromised people stay safe.
- Vaccination – It’s extra important that people who are immunocompromised stay up to date with Covid-19 vaccines.
- Treatment – If you’re immunocompromised and get Covid-19, there are treatments designed to make your case less severe.
- Research – To find new ways to protect people from Covid-19, researchers are studying new medicines and vaccines that may help immunocompromised people stay safe.
Why should immunocompromised people participate in clinical trials?
- Most previous research into Covid-19 treatments and vaccines did not include people who are immunocompromised.
- By joining a Covid-19 study, you can help answer key questions about how to protect people with weakened immune systems.
- You may get medicine that could help protect you from the effects of Covid-19.
Are clinical trials safe for immunocompromised people?
Most clinical trials are carefully monitored by an Institutional Review Board or IRB. IRBs protect participants’ rights and make sure research studies are ethical and appropriate.
How to find a clinical trial for Covid-19 treatment
Clinicaltrials.gov
NIAID Covid-19 Studies
Study Understanding Pre-Exposure pRophylaxis of NOVel Antibodies (SUPERNOVA)
A Nasal Treatment for Covid-19
A Study to Evaluate EDP-235 in Non-Hospitalized Adults with Covid-19 (SPRINT)
This educational resource was created with support from AstraZeneca.
Resources
AstraZeneca COVID-19 Clinical Trial Materials for Immunocompromised People
Español
- ¿Es seguro participar en un ensayo clínico si eres inmunodeficiente?
- Soy inmunodeficiente y un ensayo clínico cambió mi vida
COVID-19 Clinical Trials
- A study for the protection of Covid-19 in people with impaired immune system (SUPERNOVA)
- A Nasal Treatment for Covid-19
- A study to Evaluate EDP-235 in Non-hospitalized Adults With COVID-19 (SPRINT)
Clinical Trials for Immunocompromised People
- We Need More Black Women in Clinical Trials ›
- HealthyWomen Is a Proud Convener on the COVID-19 Vaccine Education and Equity Project (CVEEP) ›
- I Was Immunocompromised and a Clinical Trial Changed My Life ›
- Is It Safe to Join a Clinical Trial If You’re Immunocompromised? ›
- COVID-19 and the Growing Importance of Diverse Participation in Clinical Trials ›
- WomenTalk: Choose to Protect - HealthyWomen ›
- A Guide for High-Risk Immunocompromised Individuals - HealthyWomen ›