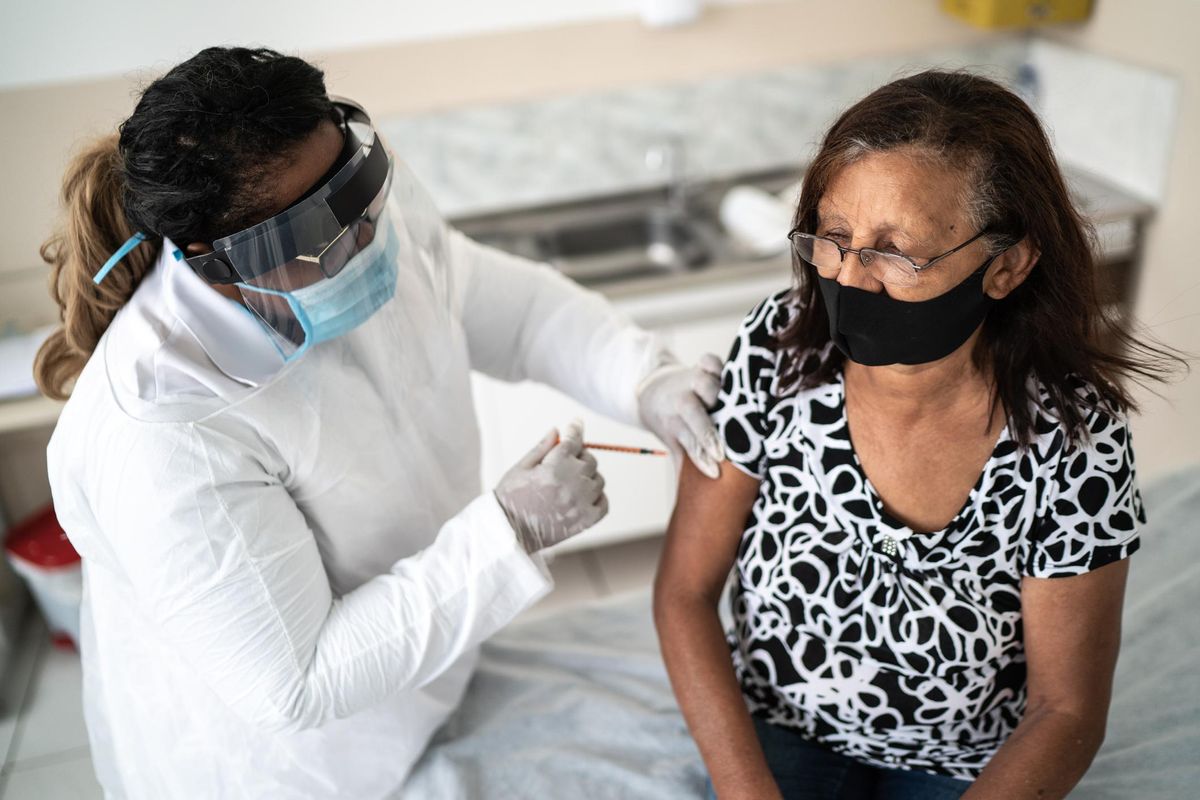
Now that COVID-19 vaccines have been authorized for use by the FDA, many questions surround their risks. The Pfizer-BioNTech and Moderna vaccines are currently available in two-dose vaccines, while Johnson & Johnson has just submitted its one-dose vaccine to the FDA for regulatory approval.
As these vaccines are being rolled out across the country, a handful of common side effects have been reported. Since side effects are one of the top concerns people have regarding the safety of COVID-19 vaccines, it's important to discuss them. However, it's important to note, the COVID-19 vaccines have been determined to be safe and effective.
Common side effects that have been reported after receiving a COVID-19 vaccine are pain and swelling in the arm where the shot was given along with fever, chills, fatigue and headaches. While these side effects can be uncomfortable, they're actually a good thing. They show that your body is responding to the vaccine and is learning how to build protection against the virus.
Most of these side effects are minor and can be soothed with over-the-counter remedies like ibuprofen, acetaminophen, drinking plenty of fluids, and applying a cool, clean washcloth to the arm that received the vaccine. These symptoms typically last a few days and then disappear.
While discomfort from fever or pain is normal, people who get the COVID-19 vaccine are advised to call their doctor or healthcare provider if side effects are extreme or don't go away after a few days. Also, if redness or tenderness at the vaccination site increases after 24 hours.
Severe side effects are rare
Although severe reactions to the COVID-19 vaccine are possible, like they are with any vaccine, they don't happen very often, and the COVID-19 vaccine has been largely tolerated well across the country.
Between December 14 and 23, 2020, only 21 cases of anaphylaxis (or severe allergic reaction) were reported out of nearly 2 million doses of the Pfizer-BioNTech vaccine. Most of these severe side effects presented themselves in the first 15 minutes after getting the vaccine, leading to prompt medical intervention.
Out of an abundance of caution, everyone who receives a COVID-19 vaccine is monitored for at least 15 minutes before they leave the vaccination location. In the event of an adverse reaction, the vaccine provider immediately sends a report to the Vaccine Adverse Event Reporting System (VAERS). It's part of a thorough reporting system in place to help monitor side effects and is one of the most intensive safety monitoring efforts in U.S. history.
Unexpected adverse reactions are quickly addressed and studied to assess whether the reaction is a risk for others. This can also help experts decide whether the U.S. recommendations for that vaccine will need to be changed to reflect more serious side effects that were not seen in clinical trials. Monitoring side effects allows the FDA and the Centers for Disease Control and Prevention (CDC) to ensure that the benefits continue to outweigh the risks for people who receive a COVID-19 vaccine.
How to self-report COVID-19 vaccine side effects
The best way to improve public knowledge of potential COVID-19 vaccine side effects is to self-report them. Everyone who receives the COVID-19 vaccine is encouraged to report any side effects to the CDC and is given a card with information on how to do so. Getting this data is essential for scientists to understand the vaccine effectiveness and overall impact.
It's especially important for women and minorities to take an active role in reporting side effects so the medical community can appropriately determine whether there are sex and/or race differences in response to the vaccine. This can lead to more personalized approaches for vaccine administration and symptom monitoring.
One way to self-report COVID-19 vaccine side effects is through V-safe, a smartphone-based tool that uses text messaging and web surveys to monitor how recipients feel after receiving the shot. This is a great way to quickly alert the CDC to any adverse reactions. The more people weigh in with their experiences, the more our knowledge about vaccine tolerance will expand.
Vaccine recipients can also use the FDA Adverse Event Reporting System, or FAERS Public Dashboard. Both healthcare providers and the general public can use this system to report any adverse reactions for drug and biologic products, including the COVID-19 vaccine. It's an easy program to use and anyone making a report can simply search "COVID-19 vaccine" and follow the instructions.
Currently, there is still limited data on how COVID-19 vaccines affect pregnant women and those breastfeeding, so this is one key area women can play a role. The reporting of any information on COVID-19 vaccine by women, pregnant women and minorities will help the medical community better research any potential differences in adverse reactions.
This knowledge then leads to the development of safer vaccines, side effect monitoring systems and guidelines for who should receive the vaccine and how. Our public health ultimately depends on us — the general public.
- Covid-19 Vaccines and False-Positive Mammograms: When Should You Get Screened? ›
- 6 Common Concerns of Vaccine-Hesitant People ›
- If I have allergies, should I get the coronavirus vaccine? An expert ... ›
- When Your Chance for a Covid Shot Comes, Don't Worry About the ... ›
- Options for Covid Vaccines - HealthyWomen ›